Abstract
Cytopenia is common in cause for considering the diagnosis of myelodysplastic syndrome (MDS), especially in the elderly population. To diagnose the presence or absence of MDS, bone marrow biopsy is performed on most patients with cytopenia. Performing bone marrow biopsy is a painful procedure and can be associated with bleeding and other complications. Moreover, bone marrow morphology is unreliable for the diagnosis of MDS. Cytogenetics and molecular studies can provide conclusive evidence for the diagnosis of MDS. Liquid biopsy and testing for molecular abnormalities in cfDNA in peripheral blood plasma is significantly less invasive than traditional bone marrow testing. We evaluated the diagnostic value of liquid biopsy in determining the presence of MDS in 640 patients presented in community setting practice with cytopenia.
Methodology: A total of 640 peripheral blood samples from patients with cytopenia were submitted for cfDNA testing. We used the TruSight Myeloid panel (Illumina, San Diego, CA) for detecting missense mutations and fragment length analysis (FLA) for detecting ITD in FLT3 and large indels in CALR . DNA was extracted from samples using the QIAamp DNA Mini Kit. This next generation sequencing (NGS) testing covers mutations in 54 myeloid-related genes. The average depth of sequencing was 10,000x.
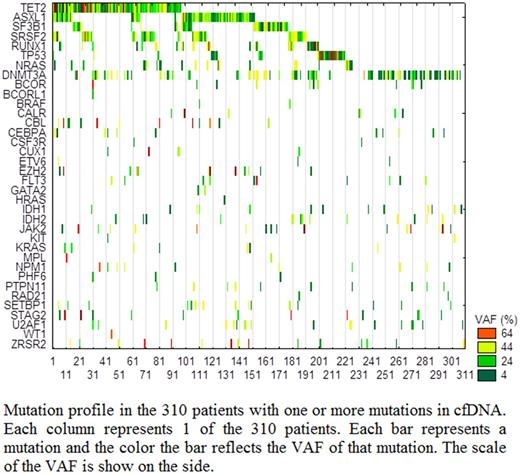
Results: Of the 640 tested patients, 310 (48.4%) showed mutations in one or more genes: 38% of these had mutations in one gene, 25.5% had mutations in two genes, and 36.5% had mutations in more than two genes. However, 14% of cases with mutation in one gene had two or more mutant subclones. The median age of all patients was 69 (range: 15-99) and 55% were males. Patients with mutations were significantly older (75 vs. 66 years old, P<0.0001) and more likely to be males (60% vs. 46% males, P=0.004). The most commonly mutated genes were TET2, DNMT3A, ASXL1, SRSF2, SF3B1, and RUNX1 detected in 31%, 28%, 25%, 17%, 15%, and 10% of the patients with mutations, respectively. Interestingly, 32 patients (10% of patients with any mutation) had mutations in TP53, 3% had mutations in FLT3, and 4% had mutations in NPM1 genes, indicating aggressive or acute disease. The average variant allele frequency (VAF) was <10% in 22% of cases with any mutation, between 10-20% in 19% of cases, between 20-30% in 16% of cases, and >40% in 43% of the cases. There was a significantly higher number of mutated genes in patients with average VAF >20% (P<0.0001). The combination of VAF, type of mutated gene, and number of mutated genes were considered in determining the significance of abnormal neoplastic clone and recommendation for management. The presence of mutations in NPM1, FLT3, TP53 as well as SF3B1 were considered diagnostic irrespective of the VAF. For patients with VAF at <20% in genes such as TET2, DNMT3A, and ASXL1 were not considered diagnostic for clinically significant myeloid neoplasm.
Conclusions: Liquid biopsy for evaluating patients with pancytopenia can be used to evaluate the presence of abnormal hematopoietic neoplasm clones and the severity of the disease. Liquid biopsy can reduce the need for bone marrow biopsy in more than 50% of patients presenting with cytopenia. VAF, mutated gene types, and the combination of mutated genes should be considered for making management decisions based on liquid biopsy.
Ma: NeoGenomics: Employment. De Dios: NeoGenomics: Employment. Funari: NeoGenomics: Employment. Jiang: NeoGenomics: Employment. Agersborg: NeoGenomics: Employment. Hummel: NeoGenomics: Employment. Blocker: NeoGenomics: Employment. Albitar: NeoGenomics: Employment.
Author notes
Asterisk with author names denotes non-ASH members.